Introduction:
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potentially curative therapeutic strategy to cure a large number of hematologic diseases. Acute graft-versus-host disease (aGVHD) remains a major obstacle against long-term survival for patient underwent allo-HSCT. Oxidative stress can generate large amounts of oxygen free radicals affecting the metabolism, proliferation, and differentiation of normal cells. It may also further induce the autoimmune response to form a waterfall effect, causing reversible to extracellular insults. It has been confirmed that oxidative stress plays an important role in the pathogenesis of aGVHD and the following organ injury. Multiple immunosuppressant agents including calmodulin inhibitor, corticosteroids, anti-CD25 antibody, and JAK1/JAK2 inhibitors have been used to treat GVHD in clinical scenarios. However, quite an amount of patients will develop to glucocorticoid-refractory aGVHD and too intensive immunosuppressive therapy will increase the incidence of infection and malignancy relapse. Nrf2/HO-1 (Nuclear factor E2-related factor 2/ Haemoxygenase-1) signaling pathway has been recognized as a major regulator against oxidative stress injury. Recent studies have demonstrated that hyperbaric oxygen therapy (HBOT) significantly improved non-healing ulcers secondary to GVHD and hemorrhagic cystitis after HLA-mismatched allo-HSCT whether induced by infection or aGVHD. Based on the important role of oxidative stress in the process of aGVHD, we hypothesis that HBOT can improve aGVHD via up-regulating Nrf2/HO-1 pathway.
Methods:
By using an allo-HSCT murine model of C57BL/6 (H-2KB)→BALB/C (H-2KD), we evaluated the therapeutic effects of HBO on aGVHD. The murine model of aGVHD was established in BALB/C mice by lethally body irradiation, followed by 1×107 bone marrow cells and 2×107 spleen cell transplantation. Mice were randomly divided into three groups: BMT+HBO group, GVHD group, and GVHD+HBO group. BMT+HBO group and GVHD+HBO group mice were simultaneously treated with HBO per day for 2 weeks from day -7 to +7 before- to post stem cell transfusion. The HBO therapy was performed for mice in a sealed chamber at a pressure of 2.4 atmosphere absolute (ATA) for 90 min per day. The induction of the murine GVHD process and GVHD scores were calculated according to the method of Cooke et al (1996) every 6 days.
Results:
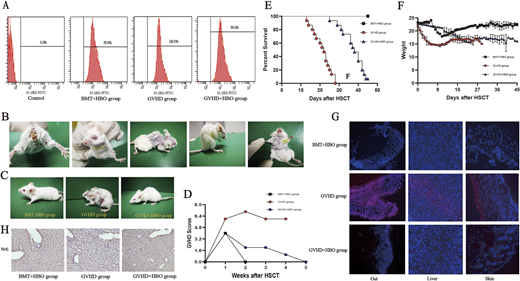
The flow cytometry showed that the level of H-2KB positive cells in the bone marrow cavity of the recipient mice was more than 95%, indicating successful implantation (Figure A). The BALB/C recipient mice in the control group (non-HBOT) showed typical aGVHD symptoms within 20 days, mainly including wasting, ruffled fur, hunched back, skin defect, ocular signs, diarrhea, and anal swelling (Figure B). The aGVHD+HBO group only showed slightly ruffled fur on 40 days post-transplantation (Figure C) and the aGVHD score of aGVHD+HBO group was much lower than that of GVHD group (Figure D)
Weight loss was mild in the aGVHD+HBO group than that in the aGVHD group (Figure E). All aGVHD group mice eventually succumbed within 1 month after transplantation, the survival was shorter than that of aGVHD+HBO group. The survival time was analyzed by Kaplan-Meier method (Figure F). The BMT+HBO group served as a control for HBO toxicity.
Immunofluorescence microscopy evaluation of mouse liver, skin, and small intestine revealed an attenuation of fluorescence signal in the aGVHD+HBO group, indicating HBO can significantly reduce ROS levels (Figure G).
Immunohistochemical staining showed that the expression of NRF2 protein in the liver collected in day 16 post-transplantation was higher than the BMT+HBO group and aGVHD group (Figure H).
Conclusions:
By using this murine model of aGVHD following allo-HSCT, our results indicated that ROS inhibition by HBOT markedly reduced the infiltration of inflammatory cells and tissue damage to targeted organs, resulting in significantly improved symptoms and prolonged survival. In conclusion, our study provided laboratory evidence that HBO can prevent and treat aGVHD by upregulating the expression of NRF2 and HO-1. HBOT might be a promising prophylactic and pre-emptive treatment choice for aGVHD. In the future, we will further investigate the accurate mechanism of HOBT in the process of aGVHD via Nrf2/HO-1 pathway, verify our preliminary animal experimental results in clinical application.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal